Genetic Testing Urged for Patients With Early-Onset Colorectal Cancer
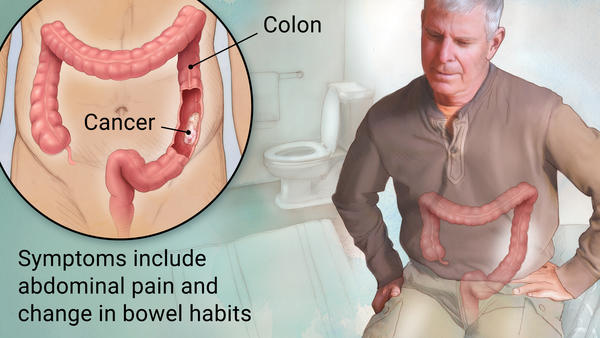
NEW YORK (Reuters Health) – Genetic counseling and testing with a multigene panel should be considered for all patients with early-onset colorectal cancer (CRC), who are at risk for a “spectrum” of hereditary cancer syndromes, according to the Ohio Colorectal Cancer Prevention Initiative Study Group.
“Hereditary cancer syndromes infer high cancer risks and require intensive cancer surveillance, yet the prevalence and spectrum of these conditions among unselected patients with early-onset CRC is largely undetermined,” Dr. Heather Hampel of The Ohio State University Comprehensive Cancer Center in Columbus and colleagues write in JAMA Oncology, online December 15.
To investigate, they recruited 450 patients diagnosed with CRC before age 50 from 2013 to 2016. Mismatch repair (MMR) – a system within the cell for detecting and correcting errors in DNA – was determined by immunohistochemistry and/or microsatellite instability. Next-generation sequencing was used to test germline DNA for mutations in 25 cancer susceptibility genes.
A total of 75 gene mutations were found in 72 patients (16%). Forty-eight patients (10.7%) had MMR-deficient tumors, and 40 (83.3%) had at least one gene mutation: 37 had Lynch syndrome (with multiple variants); one patient had the APC c.3920T>A, p.I1307K mutation and a PMS2 variant; nine patients (18.8%) had double somatic MMR mutations; and one patient had somatic MLH1 methylation.
A total of 402 patients (89.3%) had MMR-proficient tumors, and 32 (8%) had at least one gene mutation: nine had mutations in high-penetrance CRC genes; 13 had mutations in high- or moderate-penetrance genes not traditionally associated with CRC (three in ATM; one in ATM/CHEK2; two in BRCA1; four in BRCA2; one in CDKN2A; and two in PALB2); and 10 patients had mutations in low-penetrance CRC genes.
“Importantly,” the authors write, “24 of 72 patients (33.3%) who were mutation positive did not meet established genetic testing criteria for the gene(s) in which they had a mutation.”
Dr. Anton Bilchik, chief of medicine and of gastrointestinal research at John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California, said the findings are important “as new drugs are (being) developed which may target these mutations.”
“Furthermore,” he told Reuters Health by email, “consideration should be given to screen other family members for colon cancer at a younger age . . . (and) every patient diagnosed with colon cancer under age 50 regardless of whether they have a family history.”
– See more at: https://www.mdalert.com/news